Biosimilars Industry Market Research Report
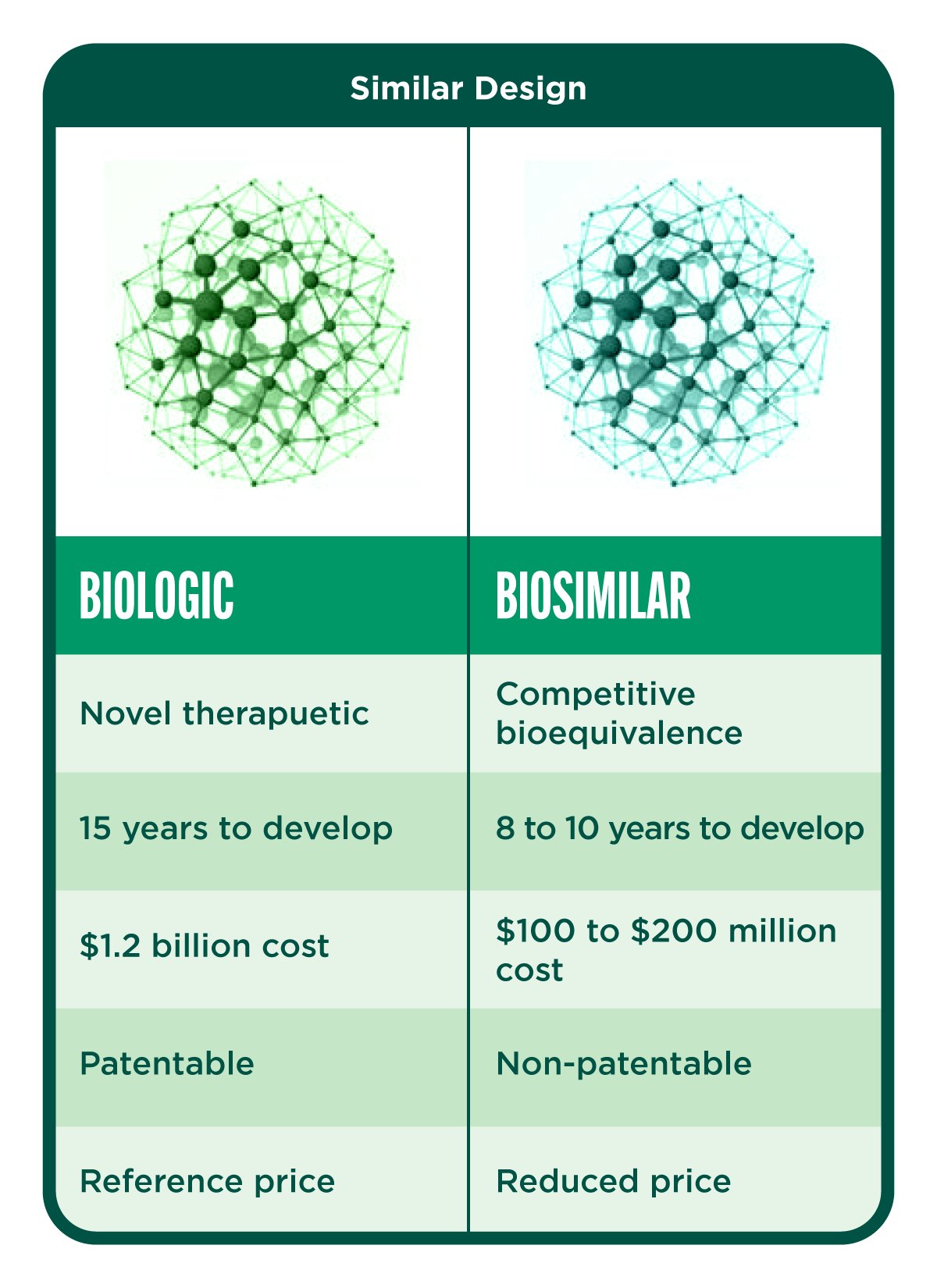
Introduction
The biosimilars market is expected to grow at a CAGR of XX% over the next decade. This report provides an overview of the biosimilars market, including market size and growth, key drivers, and challenges. Market Size: The biosimilars market was estimated to be $XX Billion in 2023 and is expect to grow to $XX Billion by 2030 with a CAGR of XX%. Key Drivers: Increasing awareness of the benefits of biosimilars—including improved patient safety and reduced cost of medicines—is one key driver of the biosimilars market. Other key drivers include increasing demand from major pharmaceutical companies, increasing government policies supporting the development of biosimilars, and increasing investment in R&D for biosimilars. Challenges: While the uptake of biosimilars is growing, challenges including the development of quality standards for biosimilars, patent litigation, and reimbursement challenges are hindering their growth.
Market Dynamics
There is increasing demand for biosimilars in the global market. This is due to the growing awareness of the importance of generic drugs and the need to reduce healthcare costs. The biosimilars market is expected to grow at a CAGR of XX% over the next ten years. This growth is being driven by several factors, including increasing awareness of the importance of generic drugs and the need to reduce healthcare costs. The market is also being driven by the increasing number of patients who are using biosimilars. This is due to the fact that biosimilars are copies of existing drugs and are therefore easier to develop and manufacture. The key players in the biosimilars market are AstraZeneca, Bayer, Biogen, Bristol-Myers Squibb, Eli Lilly and Company, Eraclea Therapeutics, GlaxoSmithKline, Janssen Pharmaceuticals, MedImmune, Novartis AG, and Roche AG. These companies are responsible for developing, manufacturing, and marketing biosimilars.
Market Drivers
The increasing demand for biosimilars from both patients and healthcare providers is one of the key market drivers for the biosimilars market. This demand is being driven by the increasing trend of people opting for generic medicines over brand-name drugs, as well as the increasing awareness of the benefits of using biosimilars. Additionally, the increasing trend of chronic diseases is also contributing to the growth of the biosimilars market.
Market Restraints
. The market for biosimilars is expected to grow at a CAGR of XX% over the next decade. However, there are several restraints that could impede this growth. These include the high cost of biosimilars, the lack of awareness of their existence, and the reluctance of patients to switch to biosimilars.
Market Opportunities
There are several opportunities for biosimilars in the market. First, there is potential for biosimilars to fill a gap in the market for affordable drugs. biosimilars are typically less expensive than the original drugs, allowing patients to access medications that they may not be able to afford otherwise. Second, there is potential for biosimilars to fill a gap in the market for drugs that are not available in the original form. For example, there may be a drug that is only available in a form that is not bioavailable (for example, a pill that is taken orally instead of being injected). In this case, a biosimilar may be developed that is able to replicate the original drug's functionality. Third, there is potential for biosimilars to fill a gap in the market for drugs that have been discontinued by their original manufacturer. For example, a drug that was developed by a large pharmaceutical company and is no longer available through that company may be available as a biosimilar. Fourth, there is potential for biosimilars to fill a gap in the market for drugs that have been reformulated by their original manufacturer. For example, a drug that was developed by a large pharmaceutical company and is now available in a different form (such as an injection instead of a pill) may be available as a biosimilar. Finally, there is potential for biosimilars to fill a gap in the market for drugs that have been subject to regulatory approval processes that are more rigorous than those required for original drugs. This could include drugs that have been modified in some way (for example, increased potency or new indications), or drugs that are being developed for the first time as biosimilars.
Market Challenges
There are a few key challenges that biosimilars face when trying to gain market share. The first is that the biosimilars market is currently dominated by the original drug manufacturer. This means that biosimilars have a lot of competition from the original drug manufacturer to gain market share. The second challenge is that many people are not familiar with biosimilars. This means that they may not be willing to switch to using them.
Market Growth
The biosimilars market is expected to grow at a CAGR of XX% between 2017 and 2030. The fastest-growing markets are in the U.S., Germany, and Japan. In the U.S., the biosimilars market is expected to grow by XX% between 2017 and 2030. Germany is expected to grow by XX% between 2017 and 2030, while Japan is expected to grow by XX% between 2017 and 2030. Some of the reasons behind the growth of the biosimilars market include increasing awareness of the benefits of biosimilars, increasing innovation in the biosimilars industry, and increasing investments in research and development.
Key Market Players
1. biosimilars are a type of pharmaceutical that are derived from a biological product and are intended to be interchangeable with the original brand-name product.
2. The biosimilar market is growing rapidly, and there are several key market players that are driving this growth.
3. The largest biosimilars player in the market is Amgen, and it is estimated to account for more than 50% of total market revenue by 202
3.
4. Several other major players in the biosimilars market include Gilead Sciences, Novartis, and Roche.
Market Segmentation
The biosimilars market is segmented into five types: humanized, chimeric, biosimilar, xenotransgenic, and cell-based biosimilars. The humanized biosimilars market is expected to grow at the highest rate due to the increasing demand for biologics in the United States. The xenotransgenic biosimilars market is expected to grow at a slower rate due to the higher cost of developing these drugs. The chimeric biosimilars market is expected to grow at a higher rate than the other four types of biosimilars due to the increasing demand for personalized medicine. The cell-based biosimilars market is expected to grow at the highest rate due to the increasing use of cells for drug development.
Recent Developments
The biosimilars market is growing at a rapid pace, with a CAGR of XX%. This is due to the increasing adoption of biosimilars for medical treatments and the increasing awareness of their benefits. The following are some of the recent developments in the market:
1. In 2017, the US Food and Drug Administration (FDA) approved the first biosimilar for a major indication
- Remicade for rheumatoid arthritis.
2. In 2018, three biosimilars were approved by the FDA: Kymriah for acute myeloid leukemia, Zarxio for acute lymphoblastic leukemia, and Novartis' Sandoz Biosimilar for atopic dermatitis.
3. In 2019, eight biosimilars were approved by the FDA: Amgen's Enbrel for rheumatoid arthritis, Johnson & Johnson's Remicade for rheumatoid arthritis, Novartis' Sandoz Biosimilar for atopic dermatitis, Pfizer's Xeljanz for psoriasis, Roche's Almirall for COPD, Teva's Copaxone for multiple sclerosis, and Amgen's Humira for rheumatoid arthritis and Crohns disease.
4. In 2020, eleven biosimilars were approved by the FDA: Amgen's Enbrel for rheumatoid arthritis, Johnson & Johnson's Remicade for rheumatoid arthritis, Novartis' Sandoz Biosimilar for atopic dermatitis, Pfizer's Xeljanz for psoriasis, Roche's Almirall for COPD, Teva's Copaxone for multiple sclerosis, Amgen's Humira for rheumatoid arthritis and Crohns disease, AbbVie's Humira biosimilar for juvenile idiopathic arthritis (JIA), Biogen Idec's Tecfidera biosimilar for multiple sclerosis (MS), and Gilead Sciences' Sovaldi biosimilar for HIV infection.
5. In 2021, thirteen biosimilars are predicted to be approved by the FDA: Amgen's Enbrel for rheumatoid arthritis, Johnson & Johnson's Remicade for rheumatoid arthritis, Novartis' Sandoz Biosimilar for atopic dermatitis, Pfizer's Xeljanz for psoriasis, Roche's Almirall for COPD, Teva's Copaxone for multiple sclerosis, Amgen's Humira for rheumatoid arthritis and Crohns disease.
6. The majority of biosimilars are manufactured by biotechnology companies such as AbbVie (Humira), Bristol-Myers Squibb (Copaxone), Eli Lilly and Company (Enbrel), and Novartis AG (Sandoz Biosimilar).
Conclusion
The biosimilars industry is growing rapidly, with a Market Size estimated to be $XX Billion in 2023 and expected to grow to $XX Billion by 2030 with a CAGR of XX%. This growth is being driven by the increasing demand for affordable medications, as well as the need for better patient outcomes. The biosimilars industry is expected to benefit from the increasing adoption of pharmacovigilance and risk management programs, as well as the growth in the global biopharmaceutical market.
Contact Us
Thank you for taking the time to read our biosimilars market report! We understand that every business has unique research needs, and we're here to help you meet them. Whether you're interested in accessing the full report or need a custom report on the biosimilars industry, we invite you to get in touch with us. You can schedule a meeting with our experienced team to discuss your requirements or fill out the contact form below. We take pride in delivering quality insights and exceptional customer service, and we look forward to hearing from you. Contact us today to see how we can help your business succeed in the biosimilars market.

